Overview
Under the direction of Jason D. Allen, PhD, the laboratory investigates vascular health and the atherosclerotic process. We combine physiological and biochemical techniques in an attempt to better detect markers of vessel disease development.
Dr. Allen is also a registered vascular specialist (RVS) through Cardiovascular Credentialing International.
Our major focus is peripheral blood flow, endothelial function, and nitric oxide (NO) bioavailability.
Under normal (healthy) conditions, NO has a vaso-protective role. Changes in the bioavailability or chemical species of NO may have a detrimental effect on vascular health.
Efforts to determine markers of NO bioavailability and vascular function may help determine who has the switch for vascular disease turned on or off.
We also investigate the outcomes of different treatment interventions on the vascularture. Exercise training as treatment for vascular disease is a specific interest of ours.
We provide an advanced vascular prevention screening service to the Duke Executive Health Program, the Duke Diet and Fitness Center, and select endocrinology patients.
Our research mission is supported by studies funded by the National Institutes of Health, the American Diabetes Association, and the American Heart Association. We are also involved in several industry-sponsored trials (see research projects).
Publications
Recent Publications
Book Chapters
- Allen, J. D. (Consultant). Physical Activity Guidelines Advisory Committee. - Physical Activity Guidelines Advisory Committee Report, Part G. Section 2: Cardiorespiratory Health 2008. U.S. Department of Health and Human Services, Washington DC. 2008. http://www.health.gov/paguidelines/
Recent Articles
- Jones LW, Fels DR, West M, Broadwater G, Allen, JD, Barry WT, Wilke LG, Masko E, Douglas PS, Dash RC, Povsic TJ, Peppercorn J, Marcom PK, Blackwell KL, Kimmick G, Turkington T, Spector NL, Dewhirst MW. (2012). Modulation of circulating angiogenic factors and tumor biology by aerobic training in breast cancer patients receiving neoadjuvant chemotherapy. Cancer Cell. In review.
- Inrig, J., Kim, C., Van Buren, P., Allen, JD., Povsic, T., Szczech, L., Vongpatanasin, W. (2012). A Pilot Study Testing the Effect of Active Vitamin D on Endothelial Cell Function and Arterial Stiffness in Hemodialysis Patients. the Clinical Journal of the American Society of Nephrology. In review.
- Robbins, JL.*, Allen, JD.*, VanBruggen, MD., Credeur, DP., Hollis, BC., Johannsen, NM., Earnest, CP., Pieper, CF., Johnson, JL., Church, TS., Ravussin, E., Kraus, WE., Welsch, MA. (*=joint 1st authors). (2012). Unlocking the Barriers to Improved Functional Capacity in the Elderly: Rationale and Design for the Fit for Life Trial. The Journal of Gerontology: Medical Sciences. In review.
- Ferguson, SK., Hirai, DM., Copp, SW., Holdsworth, CT., Allen, JD., Jones, AM., Musch, TI., Poole, CD. (2012). Impact of nitrate supplementation via beetroot juice on exercising muscle vascular control in rats, Journal of Physiology. In review.
- Allen, JD., Giordano. T. and Kevil, CG. (2012). Nitrite and Nitric Oxide Metabolism in Peripheral Artery Disease (Review). Nitric Oxide Biology and Chemistry special topics edition “Nitrite and Nitrate Bench to Bedside.” 26(4), 217-22. PMID: 22426034
- Lin, PH., Allen, JD., Li, YJ., Lien, LF., Svetkey, LP. (2012) Blood Pressure Lowering Mechanisms of the DASH Dietary Pattern. Journal of Nutrition and Metabolism. 2012:472396. Epub 2012 Jan 30. PMID: 22496969
- Lane, WO., Jantzen, AE., Jamiolkowski, RM., Haseltine, JM., Galinat, LJ., Lin, F., Lawson, JH., Allen, JD., Truskey, GA., and Achneck, HE. (2012). Parallel-Plate Flow Chamber and Continuous Flow Circuit to Evaluate Endothelial Progenitor Cells under Laminar Flow Shear Stress. Journal of Visual Experiments, e3349.
- Duscha, BD., Robbins, JL., Jones, WS., Kraus, WE., Lye, RJ., Sanders, JM., Allen, JD., Regensteiner, JG., Hiatt, WR., and Annex, BH. . (2011). Angiogenesis in Skeletal Muscle Precede Improvements in Peak VO2 in Peripheral Artery Disease Patients. Arteriosclerosis, Thrombosis, and Vascular Biology, 31(11), 2742-2748. PMID: 21868709
- Allen, JD., (2011). Letter to the editor in response to “Supplementation with nitrate and nitrite salts in exercise: a word of caution”, Journal of Applied Physiology, 111(2), 618. PMID:21828256.
- Robbins, JL., Jones, WS., Duscha, BD., Allen, JD., Sanders, JM., Kraus, WE., Regensteiner, JG., Hiatt, WR., Annex, BH. (2011). Relationship between Leg Muscle Capillary Density and Peak Hyperemic Blood Flow with Endurance Capacity in Peripheral Arterial Disease. Journal of Applied Physiology. 111(1), 81-86. PMID: 21512146.
- Kenjale, AA., Ham, KL., Stabler, T., Robbins, JL., Johnson, JL., VanBruggen, MD., Allen, JD., Privette, G., Yim, E., Kraus, WE., and Allen, JD. (2011). Dietary Nitrate Supplementation Enhances Exercise Performance in Peripheral Arterial Disease. Journal of Applied Physiology. 110, 1582-1591. PMID: 21454745.
- Allen, JD., Ham, KL., Dumont, DM., Sileshi, B., Trahey, G.E., and Dahl, JJ. (2011). The Development and Potential of Acoustic Radiation Force Impulse (ARFI) Imaging for Carotid Artery Plaque Characterization. Vascular Medicine. 16(4), 302-311. PMID:21447606
- Achneck, A., Jamiolkowski, R., Jantzen, A., Haseltine, J., Lane, W., Huang, J., Galinat, L., Serpe, M., Lin, F., Li, M., Parikh, A., Ma, L., Chen, T., Sileshi, B., Milano, C., Wallace, C., Stabler, T., Allen, J., Truskey, G., and Lawson, J. (2011). The biocompatibility of titanium cardiovascular devices seeded with autologous blood-derived endothelial progenitor cells: EPC-seeded antithrombotic Ti Implants. Biomaterials. 32(1), 10-18. PMID: 20926131
- Jones, LW., Douglas, PS., Eves, ND., Marcom, PK., Kraus, WE., Herndon, JE., Inman, BA., Allen, JD., and Peppercon, J. (2010). Rationale and Design of the Exercise Intensity Trial (EXCITE): A Randomized Trial Comparing the Effects of Moderate Versus Moderate to High-Intensity Aerobic Training Women with Operable Breast Cancer. BMC Cancer, 10, 531. PMID: 20925920.
- Allen, JD., Stabler, T., Kenjale, A., Ham, KL., Robbins, JL., Duscha, BD., Dobrosielski, DA., and Annex, BH. (2010). Plasma Nitrite Flux Predicts Exercise Performance in Peripheral Arterial Disease following 3 months of Exercise Training. Free Radical Biology in Medicine. 49(6), pp1138-1144. PMID: 20620208.
- Stabler, T., Kenjale, A., Ham, KL., Jelesoff, N., and Allen, JD. (2010). Potential Mechanisms for Reduced Delivery of Nitric Oxide to Peripheral Tissues in Diabetes Mellitus. Annals of the New York Academy of Sciences. Vol 1203, pp101-106
- Lou, J., Povsic, T.J., Allen, J.D., Myles, S., Starr, A.Z., Ortel, T.L., and Becker, R.C. (2010). The Effect of Aspirin on Endothelial Progenitor Cell Biology: Preliminary Investigation of Novel Properties. Thrombosis Research. 126(3):ep175-9.
- Allen, J. D., Gow, A. J. Nitrite, NO and Hypoxic Vasodilation. (Commissioned Commentary). (2009). British Journal of Pharmacology. 158, pp1653-1654.
- Allen, J. D., Miller, E.M., Schwark, E., Robbins, JL., Duscha, BD., and Annex, BH. (2009). Plasma Nitrite Response and Arterial Reactivity Differentiate Cardiovascular Health Status and Performance. Nitric Oxide Biology and Chemistry. 20, pp:-231-237
- Dumont, D. M., Dahl, J. J., Miller, E. M., Allen, J. D., Fahey, B. F., and Trahey, G. E. (2009). Lower-limb vascular imaging with acoustic radiation force elastography: Demonstration of in vivo feasibility. IEEE Trans Ultrason Ferroelectr Freq Control, 56(5), pp931-944
- Dahl, J. J., Dumont, D. M., Miller, E. M., Allen, J. D., and Trahey, G. E. (2009). Imaging for Noninvasive Characterization of Atherosclerotic Plaques in the Carotid Artery: Feasibility Study. Ultrasound in Medicine and Biology, 35, (5), pp:-707-716.
Research Projects
Dietary Nitrate Supplmentation in Peripheral Artery Disease and Diabetes Mellitus+Peripheral Artery Disease
Peripheral artery disease (PAD) is a form of cardiovascular disease (CVD) caused by atherosclerotic occlusions in the legs. It affects approximately 5% of the US population over 50 years of age, one third of which suffer from ischmic leg pain that occurs with walking and improves with rest. We have previously shown that supervised exercise therapy is an efficacious treatment for PAD, with improvements of 66% in claudication pain onset time and 17% in peak walking time after 3 months of supervised training. Despite these improvements, claudication pain is still the major limiting factor to the intensity and duration of work that can be performed during training for daily activities.
Diminished nitric oxide (NO) bioavailibility is an underlying feature of CVD and PAD. NO plays an important role in inflammation, thrombosis, vascular tone, blood flow, tissue perfusion, and exercise capacity. Consequently, a therapy that would effectively deliver NO to the peripheral tissues that are under-perfused due to occlusive vascular disease is an area of our interested.
Our aim is to utilize the dietary supplmentation of nitric oxide (in the form of beetroot juice) as an intervention to acutely improve oxygenation to areas of ischema AND to chronically increase vessel growth to these ischemic areas. We are currently working with two populations to investigate the potential benefits of this intervention and treatment method.
- Increased Plasma Nitrite, Tissue Oxygenation and Functional Changes in PAD (1R21 1HL 111972-01)
- Dietary Nitrate to Augment Exercise Training Benefits in DM+PAD (1 R21 HL113717-01)
Incidence of PAD in subjects with Type II Diabetes is greatly increased in comparison to non-diabetic individuals. It is thought that diabetics may be less able to up-regulate endothelial NO production during acute exercise and in response to chronic training. Therefore, increasing dietary nitrite supplementation may hold significant potential as an effective, selective NO donor for acute and chronic tissue ischemia and a means of effective therapy for individuals with both diabetes and PAD.
Through these interventions, we hope to improve exercise tolerance, overall function, and quality of life for these individuals with PAD as well as contribute valuable treatment possibilities to the medical community.
Acoustic Radiation Force Imaging of Carotid Plaque Morphology and Composition NHLBI-2RO1HL075485
In conjunction with Duke biomedical engineers Gregg Trahey (PI) and Jeremy Dahl, and vascular surgeons Jeffrey Lawson and Ban Sileshi, we are developing and testing acoustic radiation force impulse (ARFI) imaging for detection of vulnerable carotid artery plaques.
ARFI imaging is a new ultrasonic imaging method that uses short duration (.03 - 1 meters per second) acoustic radiation force to generate localized displacements in tissue. These displacements are tracked using conventional ultrasound and correlation-based speckle-tracking methods. Tissue displacement is inversely related to tissue stiffness, and the recovery of tissue to its original position is related to its viscoelastic properties.
Because arterial walls, soft tissue, atheromas, and calcifications have a wide range in stiffness, they represent excellent candidates for ARFI imaging. Furthermore, because a single transducer on a diagnostic scanner is used to both generate the radiation force and track the resulting displacements, and there are thousands of installed ultrasonic scanners in vascular laboratories that could be programmed to include ARFI, this modality could quickly and inexpensively be added to current vascular diagnostic methods. The aim of this grant is to further develop the tecnology of ARFI and to evaluate the potential of ARFI imaging for the generation of high-resolution images of carotid plaque composition and detecting vulnerable plaques.
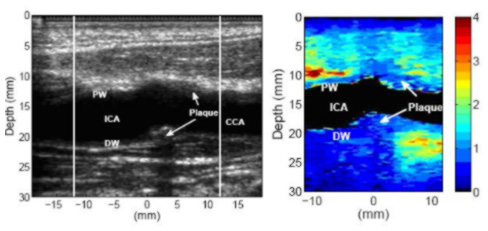
Above is a longitudinal view of a stiff, possibly fibrous, plaque in the common carotid artery of a 49-year-old male. The B-mode image indicates a plaque on the far wall of the common carotid artery. The plaque appears to wrap around a significant portion of the artery (for cross-section scans, not shown here), and is therefore visible on the near wall of the common carotid artery. The plaque appears to be homogeneously stiff throughout.
In contrast, below is an example of an apparently heterogeneous plaque containing a soft region surrounded by a stiff cap in the carotid bifurcation of a 55-year-old female volunteer. The plaque is located near the bifurcation of the carotid artery and is visible on both the proximal and distal walls. The soft region of the plaque is visible on the distal wall as the oval shaped region extending from 23 to 25 mm depth and -2 to 4 mm laterally in the ARFI image.
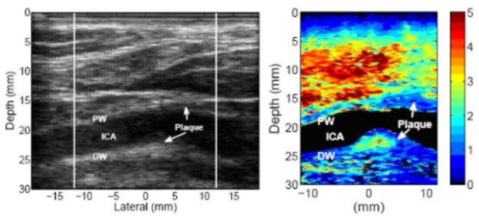
The soft region is surrounded by a significantly stiffer region. The displacement of the soft region ranges from 2.5 - 4 µm, compared to 0.5 - 1 µm in the surrounding stiffer tissue. The surrounding tissue is approximately 1.3 mm thick between the soft region and the vessel lumen, except on the left side, where the thickness of the cap decreases to approximately 0.7 mm. At 0.7 mm, the stiff cap may be considered to be at the upper limit of the range indicating vulnerability (Ge et al., 1999). Ge et al. (1999) also noted that a lipid core with area greater than 1 mm2or a core-to-plaque ratio greater than 20 percent also correlated well with rupture. The soft region in this plaque meets Ge’s definition of a vulnerable plaque, assuming that this region is a lipid core.
We are also working toward development of 3-D modeling of carotid arteries and plaques to enable us to match accurately with histology samples.
Click here for further information about our previous research studies.
Previous Research
FIT for LIFE (Mechanisms and Functional Outcomes of Exercise Progression Models in the Elderly) 5RC1AG035822-02 (10/01/09-09/30/11)
By 2030, the number of Americans 65 years old or older will account for roughly 20 percent of the U.S. population, with a projected increase in the nation’s health care spending of 25 percent. A major factor in the projected increase will be the consequences of progressive declines in functional capacity with advancing age. The decline in functional capacity can be slowed with the help of exercise training as recommended by the American College of Sports Medicine (ACSM) and American Heart Association (AHA).
Unfortunately, improvements in functional capacity in the elderly are rather small, in part because many elderly cannot tolerate exercise of sufficient intensity due to orthopaedic and cardiovascular limitations. Thus, despite some success, existing training strategies for the elderly appear less than optimal.
Therefore, the challenge for our rapidly aging nation is to define interventions for the elderly that can slow declines in functional capacity and performance and prevent early onset of physical disability and loss of independence. The potential impact is a reduction in the probability of disease, disability, early mortality, and health care expenditures.
We hypothesize that regionally specific exercise stimuli, applied at the beginning of a training program, will serve as a physiological primer capable of removing peripheral barriers that limit functional capacity in elderly at risk of losing independence. Removal of peripheral barriers early in a training program will unlock a greater potential for change in functional capacity.
Subsequently, this proposal has two objectives:
To determine differences between four weeks of a regionally specific exercise stimulus and standard aerobic exercise training on physical and functional performance
To determine the effects of subsequent eight weeks of progressive whole-body training protocol on the magnitude of change in physical and functional performance.
Of particular importance to these objectives is the combination of physiological information from biospecimens (vascular and muscular) with functional outcomes in the elderly, following two different types of exercise training progression models.
Other Projects We Are Involved In
American Diabetes Innovative Award: Effect of Hb glycation on NO metabolism and bioactivity in diabetes (07/07/08 - 06/30/2010)
1-08-CR-03 PI:- Brian Annex, MD 01/01/08-12/31/09
American Diabetes Association Clinical Research Award
Diabetes Mellitus and PAD: Mechanisms of Exercise Training
Specific Aim 1: Establish the changes in skeletal muscle measures (vascular density, oxidative metabolism/mitochondrial content, and contractile protein composition) and vascular reactivity, with objective clinical outcomes (maximal and initial pain-free walking times on treadmill testing, and peak VO2) after three weeks and 12 weeks of supervised exercise training.
W81XWH-06 PI: Lee Jones, PhD 04/1/06–03/31/09
US Department of Defense
The Effects of Exercise Training on Tumor Vascularity and Response to Neoadjuvant Therapy in Operable Breast Cancer: A Phase I-II Study
The primary aim of phase I of this study is to establish whether combining endurance exercise training with chemotherapy leads to unacceptable exercise adherence rates or unusually high DLTs. In absence of these effects, the specific aims of phase II will be to explore the effects of combining endurance exercise training with chemotherapy versus chemotherapy alone in 23 patients with locally advanced breast cancer on (i) tumor physiology (blood flow, MVD), (ii) systemic response (circulating VEGF and EPCs, endothelial function and exercise capacity), and (iii) tumor response (pathologic and clinical response).
GlaxoSmithKline Inc. PI: Lynda Szcech, MD 11/01/06-10/31/08
Endothelial Cell Dysfunction as the Mediator of Increased Morbidity and Mortality among HIV infected Patients with Proteinuria
The aim of this proposal is to test the hypothesis that proteinuria is associated with abnormal markers of endothelial cell function among HIV-infected patients. These observations will provide information describing and supporting the potential for endothelial cell dysfunction as the link between proteinuria and poorer outcomes in this population. The conclusions from this proposal will be subsequently used as a foundation to design further interventions aimed at affecting these intermediate outcomes.
AHA Grant-in-Aid PI: Pao-Hwa Lin, PhD 06/01/07–05/31/10
Mechanism(s) of the blood-pressure-lowering effects of the Dietary Approaches to Stop Hypertension (DASH) dietary pattern: DASH mechanism study
The BP-lowering effect of the DASH dietary pattern was maximal after two weeks of controlled feeding and was comparable in magnitude to antihypertensive medication among participants with stage 1 hypertension. Thus, efforts to determine the BP-lowering mechanisms of DASH should involve evaluation of hypertensive patients during the first two weeks of exposure to this dietary pattern.
PI: Jula K Inrig, MD 09/01/07-02/01/09
Effect of Hectoral on Endothelial Cell Dysfunction in ESRD
A prospective observational study describing the effect of hectoral on endothelial cell function in dialysis patients.
American Heart Association PI: Hardean E. Achneck MD 07/08/08-06/31/10
Postdoctoral Fellowship
Cell Seeding Endothelial Progenitor Cells onto Ventricular Assist Device Surface
The Biochemistry Laboratory
Overview
The biochemistry laboratory identifies metabolites of nitric oxide bioavailability and reactive oxygen species applied to endothelial and vascular health.
Nitric oxide (NO) is a simple diatomic molecule and a diffusible gas. It is unstable and has a half-life of seconds. It has been shown to play a role in the regulation of almost every major organ system.
In the vasculature, NO is synthesized by the endothelium and can also be carried by red blood cells. A simplified map of possible NO pathways in the vasculature is shown below.
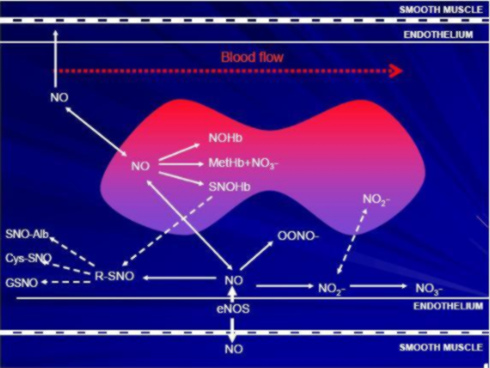
NO has several anti-atherogenic properties, including:
- Inhibition of platelet aggregation (anti-thrombotic)
- Inhibition of leucocyte adhesion to the vessel wall (anti-inflammatory)
- Inhibition of smooth muscle growth into the lumen (anti-proliferative)
- Inhibition of vasoconstrictors (vasodilation-indirect)
- Vasodilation (via shear and/or receptor mediators)
NO in the Plasma
We have investigated the effects of vascular health on NO metabolites. Plasma nitrite (NO2-) is the main oxidation product of NO and has been shown to reflect changes in eNOS activity. It therefore appears to be a good marker of recent NO bioavailability.
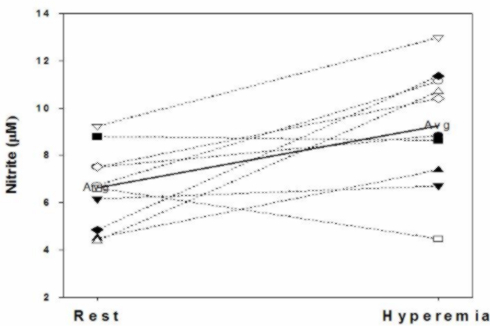
Example of a Recent Study
Abbreviations:
- RF = greater than two traditional risk factors, no clinically diagnosed CVD
- DM = type 2 diabetes no clinically diagnosed CVD
- PAD = intermittent claudication > three months and ABI<0.9 at rest.
- (In Press, Nitric Oxide: Biology and Chemistry, 2009)
We have shown plasma NO2- can be upregulated following occlusion (five minutes) and reactive hyperemia in the arm. In 15 apparently healthy subjects (34.1+7.3yrs) mean plasma nitrite concentrations were 415+64.0nM at baseline, and 634+57.1nM following reactive hyperemia. This represents a 52.5 percent increase in plasma nitrite (p=0.015). Perhaps, just as significant as the increase in the mean plasma nitrite concentration is the observation that of the 15 individuals who performed the test, the plasma nitrite level fell in only two (and in one of those by less than 5 percent). There was no change in plasma NOx (predominantly NO3-) levels.
Additionally, we have measured plasma NO2- in several different populations with varying severity of vascular disease or risk. We use acute exercise stress as a stimulus to test if individuals can upregulate NO.
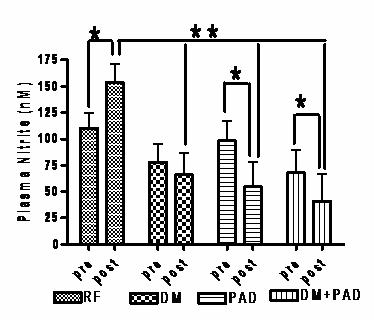
There were no differences in resting plasma nitrite values between groups. However, following the GXT, the RF group had significantly greater plasma nitrite concentrations than the other groups (after adjustment for both age and VO2peak). Furthermore, within group (pre-post) t-tests revealed a significant increase in plasma nitrite in the RF group (+39.3 percent), no change in the DM (-15.51 percent), and a significant decrease in the PAD (-44.20 percent) and PAD+DM (-39.95 percent). There were no significant changes in plasma nitrate from baseline for any of the groups following treadmill exercise.
Exercise training has been shown to improve endothelial function, so we are currently examining its effects on nitrite response to an acute exercise stress (nitrite flux) in subjects with various degrees of vascular disease. In particular we are looking at PAD and diabetic subjects. The improvement in "nitrite flux" is shown in the figure below. This was related to changes in physiological endothelial function and exercise performance.
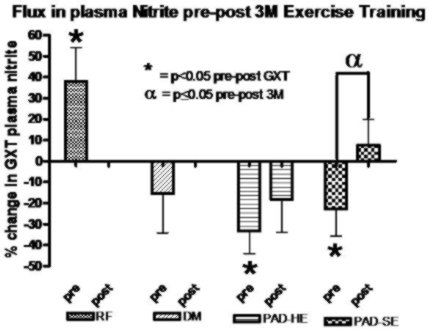
(This work is supported in part by NIHLBI grant 5RO1 HL-075752-05.)
NO in the Red Blood Cell
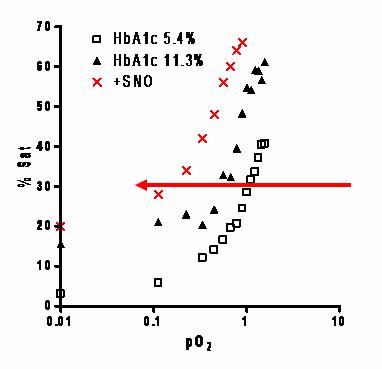
James group in Cardiff (Wales) have previously shown there may be defects in NO offloading in glycated RBC's (found in Diabetes). We have similar findings, and the pilot data opposite suggests that glycation (HbA1c11.3 percent) moves the free O2 binding curve to the left in comparison to normal Hb (A1c 5.4 percent), indicating a change from R to T state at lower pO2 and “later” O2 offloading.
We also measured the physiological responses of this glycation effect. Below is data from a single rabbit aortic ring suspension trial (three rings per condition). In the presence of glutathione (GSH), normally glycated Hb (A1c5.4 percent) dilated hypoxic vessel rings both prior to (9 percent) and following SNO loading (18.5 percent). Under identical conditions there was no response from highly glycated Hb (A1c11.8 percent). Taken together, these findings suggest that in glycated cells, NO may be "stuck" on the cell, which may effect delivery at the micro-vessels.
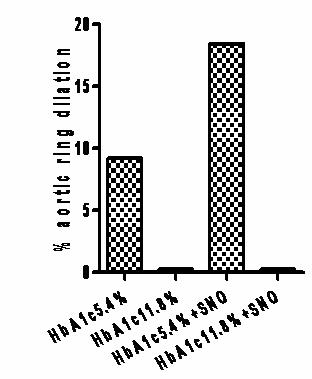
The loss of this “RBC-NO carrier” system may be very pertinent for vascular disease prevention in diabetic subjects.
This ongoing work is funded by the 7-08-IN-01 American Diabetes Association Innovative Award.
Staff
Thomas Stabler is responsible for the day-to-day running of the lab.
Contact Information
Office: Building GSRB1, Room 1033, 595 LaSalle Street, Durham, NC, 27710
Phone: 919-681-6808
E-mail: thomas.stabler@duke.edu
Vascular Lab
Overview
The vascular lab uses non-invasive techniques to assess markers of endothelial and vascular health.
We routinely perform assessments of both physiological and structural markers of vascular health for our own research projects and as a service for other investigators at Duke and externally. Some of these procedures as well as data from our research are detailed below.
Some of the procedures we employ include:
Physiological Markers
Physiological markers of vascular disease are dynamic measures that can be influenced by stress or medications. Consequently they are usually measured in the mornings following an overnight fast and other preparations.
- Brachial artery flow-mediated dilation
- Pulse wave velocity (PWV) and pulse wave reflection (PWR) analysis of vascular stiffness
- Plethysmographic limb blood flow
Structural Markers
- Carotid intimal-medial thickness (CIMT)
- Doppler ankle-brachial index (ABI)
Physiological Markers
Brachial Artery Flow-Mediated Dilation
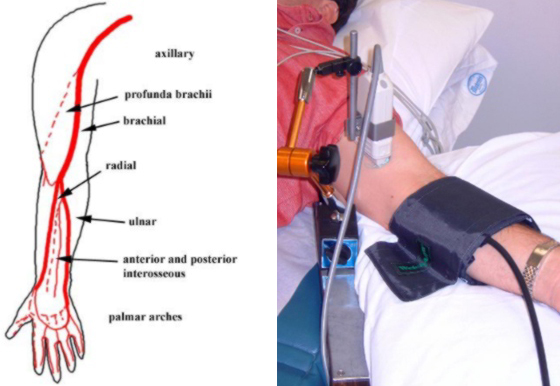
Brachial artery flow-mediated dilation (BAFMD) is a dynamic assessment of endothelial function, measured in response to forearm reactive hyperemia. It has been associated with nitric oxide bioavailability and shown to be prognostic in coronary artery disease and peripheral arterial disease. The response of the brachial artery has been shown to be similar to that of the coronary arteries but is much easier to measure and so is often used as a surrogate measure.
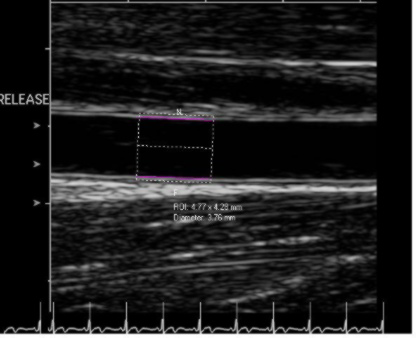
Above is a typical image of the brachial artery with specialized software tracking the vessel wall over time. We have also set image acquisition to only capture diastole images.
The figures below (left) show the diameter responses over time of healthy subjects versus subjects with CVD to the hyperemic stimulus. Notice the healthy subjects dilate over 5 percent, whereas the CVD group peak at 2 percent. This is reflected (right) in the bar graph of responses from different disease states.
(H = healthy, RF = CVD risk factor subjects, DM = type 2 diabetics, PAD = peripheral arterial disease)
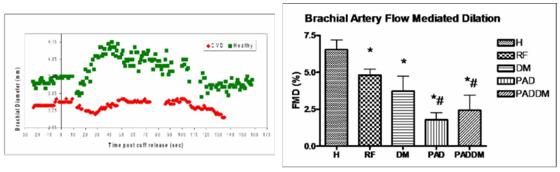
Cardiovascular disease (CVD) risk factors associated with abnormal BAFMD (and reduced NO bioavailability) include:
- Hypercholesterolemia/hyperlipidemia
- Hypertension
- Diabetes/hyperglycemia
- Aging
- Cigarette smoking
- Oxidative stress
- Sedentary lifestyle
Pulse wave velocity (PWV) and reflection (PWR) analysis of vascular stiffness
Pulse Wave Reflection
The blood pressure waveform is the sum of the pressure wave generated by the left ventricle and the pressure waves reflected from terminations and bifurcations in vascular beds.
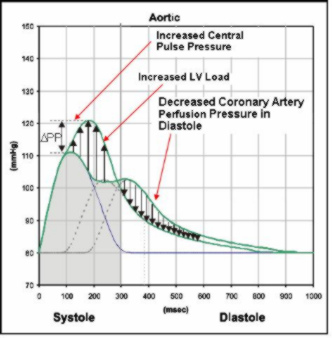
Stiffer arteries (the aorta and muscular arteries) increase the speed of the traveling pressure waves, leading to earlier return to the heart of the reflected waves.
Vasodilation and vasoconstriction of the muscular arteries changes both the size and speed of the reflected waves.
An earlier return of the reflected pressure wave changes the pressure waveform by increasing the central systolic and pulse pressures. In the aorta, the reflected wave moves also more into systole.
These changes may have important clinical implications, including:
Central pulse pressure increases, increasing risk of stroke and renal failure (increasing arrows)
Left ventricle (LV) load increases, increasing LV mass, and accelerating progress towards LV hypertrophy and heart failure (increasing arrows)
Coronary artery perfusion pressure in diastole decreases, increasing risk of myocardial ischemia (decreasing arrows)
Pulse Wave Velocity
Pulse wave velocity is a direct measure of arterial stiffness and has been shown in large populations to predict coronary artery disease and stroke. Pulse waves are waves that push against the artery walls with every heartbeat as blood moves through arteries to supply body tissues. Pulse wave velocity is the speed with which one wave or pulse travels from the heart through the arteries of the body.
Typically, the faster the pulse wave travels, the stiffer the arteries, and vice-versa. For example, a pulse wave velocity of around six metres per second (m/s) is considered highly compliant (a healthy arterial system), whereas velocities in excess of 14 m/s indicate stiffer arteries (a less healthy arterial system).
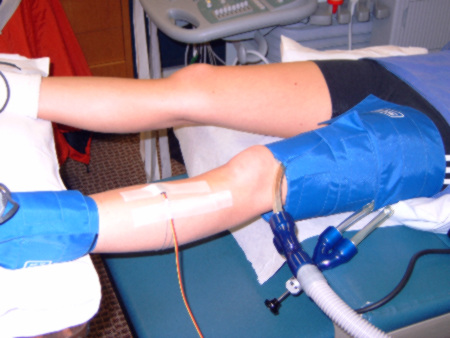
Plethysmographic Limb Blood Flow (Arterial and Venous Function)
We use strain gauge plethysmography to look at whole limb arterial inflow, venous capacitance, and maximum venous outflow. This test detects possible blockages in arteries, endothelial dysfunction, and deep venous thrombosis in the legs.
Structural Markers of Vascular Disease
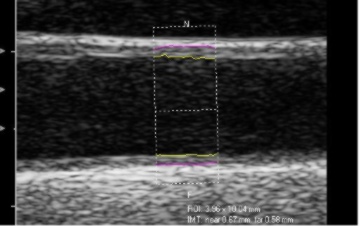
Carotid intimal-medial thickness
Carotid intimal-medial thickness (CIMT) levels have been shown to predict cardiovascular events such as heart attack and stroke.
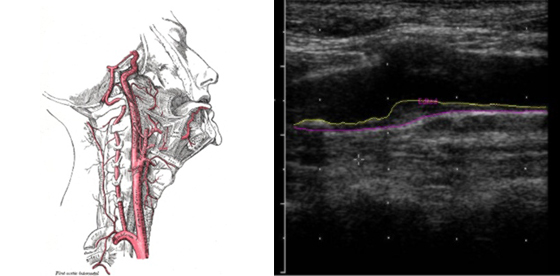
We take multiple measures of the IMT in multiple sections of the carotid arteries at several different angles of interrogation. Using specialized software to measure the artery lining and data from the Atherosclerosis Risk in Communities trial (ARIC), we can estimate the rank score of individuals’ CIMT compared with data from people with of the same age, race and gender.
CIMT Prediction Trials
Atherosclerosis Risk in Communities (ARIC) study
- 7289 females, 5552 males, 45-70 years, no history of coronary artery disese, followed four to seven years
- Hazard ratio for myocardial infarction of coronary heart disease high versus low tertiles were 6.69 for females and 2.88 for males
Cardiovascular Health Study
- 5858 subjects, older than 65 years, no apparent coronary heart disease, followed for 6.2 years
- Highest quintile = 3.87RR of myocardial infarction or stroke versus lowest
- Strong predictor even after adjustment for risk factors
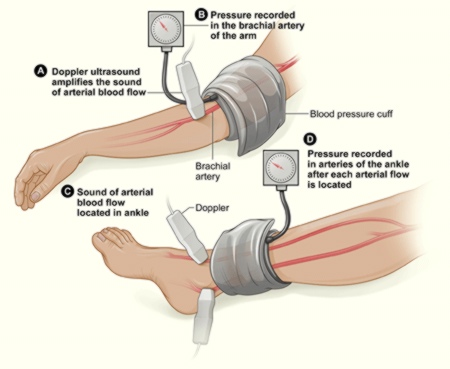
Doppler Ankle-Brachial Index (ABI)
This test is used to diagnose peripheral artery disease. It compares blood pressures in the feet with those in the arms to find out if there is a reduction in perfusion in the lower limbs. A normal ABI is 1.0 or greater (with a range of 0.90 to 1.30).
Staff
Katherine L Ham, MS
Phone: 919-660-6793
E-mail: katherine.ham@duke.edu
Mitch VanBruggen, MA
Phone: 919-660-6638
E-mail: mitch.vanbruggen@duke.edu
Contact Information
Office: The Andrew Wallace Clinic Building
3475 Erwin Road, Durham, NC, 27710