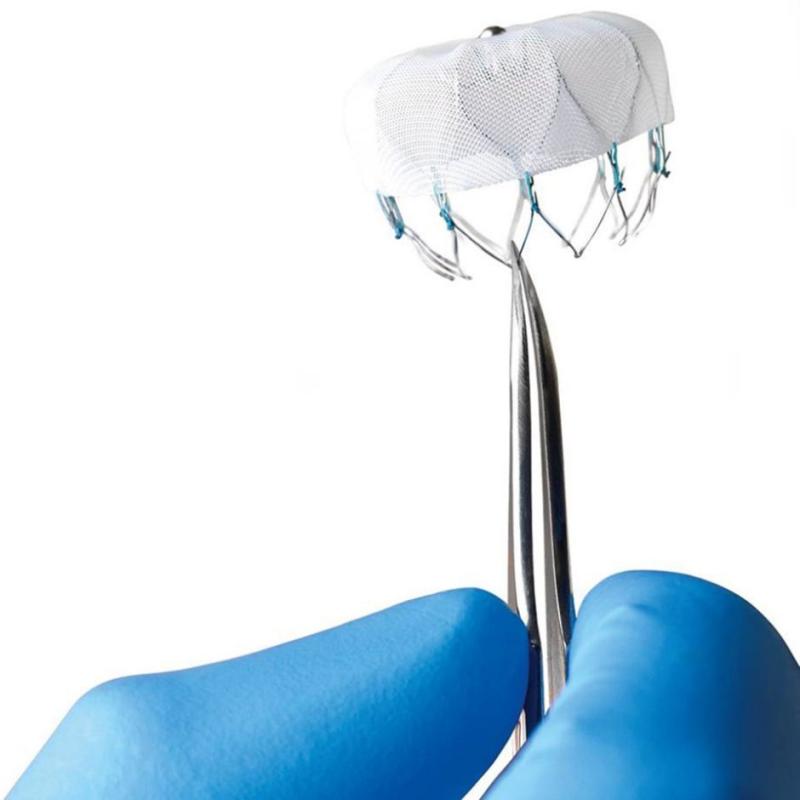
Duke University Medical Center was selected to be an early adopter site, along with two other institutions in NC (UNC and the Sanger Clinic), to implant the WATCHMAN device, which is intended to physically occlude the left atrial appendage and reduce the risk of thromboembolic stroke.
"The Duke team was the first to get a program up and running and we did their first case on Friday, May 29," said Jonathan Piccini, MD, associate professor of medicine. "It went very well."
Dr. Piccini said that, to his knowledge, Duke's case was the first FDA-approved WATCHMAN implant in the Carolinas.
“One of the reasons we were selected was our track-record with cross disciplinary collaboration and the strength of our AF center, our AF research program, extensive experience with interventional occluder devices to treat various forms of structural heart disease, and the Heart Center," said Piccini.
The Duke Heart Center's regimented, patient-centered approach to TAVR cases and lead extraction were also cited as important reasons for our selection, he said.
“We also wanted to participate as the procedure is innovative and represents a very important therapy for our patients who cannot take oral anticoagulation, yet remain at high risk of stroke."
Duke WATCHMAN implant team members include Todd Kiefer, Kevin Jackson, Zainab Samad, and Sreek Vemulapalli.